文章目录[隐藏]
2024年1月24日,中国广州中山大学肿瘤医院南方肿瘤学国家重点实验室,广东省癌症临床研究中心Liang Kang ,Huashan Liu等老师在Nature Communications(IF=16.6)上,在线发表题为“Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death”的论文,此论文使用了联科生物Annexin V-FITC试剂(货号;AP101-100-AVF),7-AAD试剂(货号;AP104-100-AAD)。
-


- AP101AVF
- 试剂盒单组分
Annexin V-FITC
- ¥520.00 – ¥1,210.00

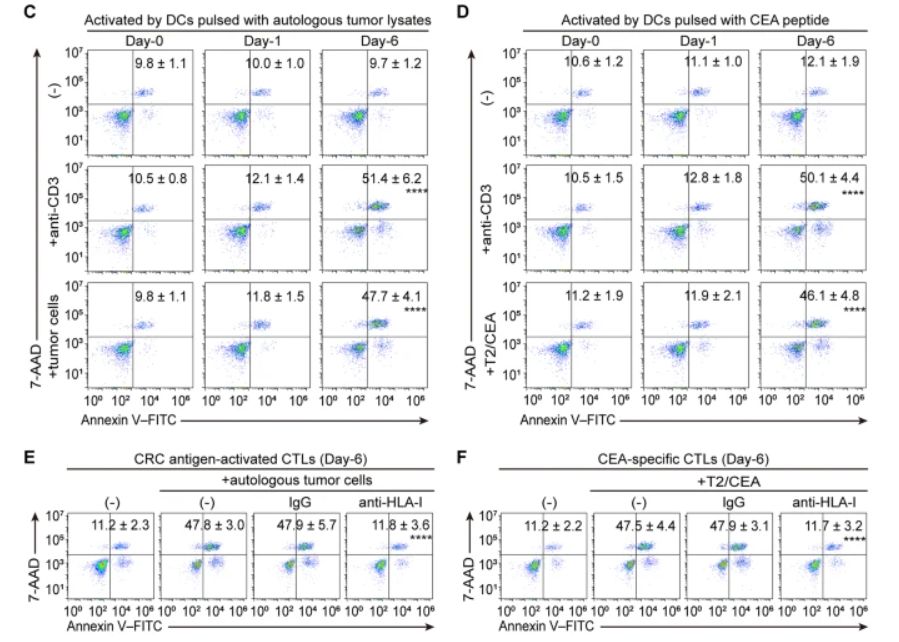
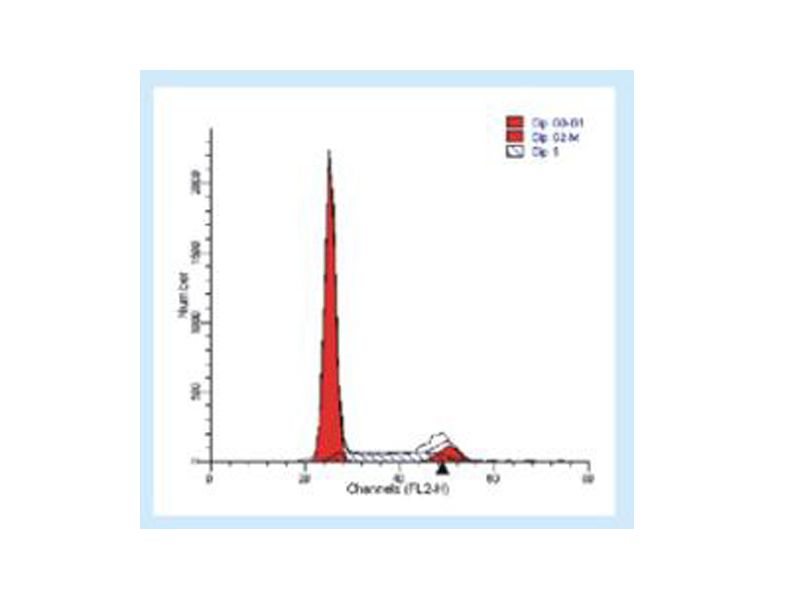
C Apoptosis of CTLs induced by anti-CD3 or autologous tumor cells (n = 4 samples; ****p ≤ 0.0001 compared with untreated day-6 CTLs by one-way ANOVA). D Apoptosis of CTLs induced by anti-CD3 or CEA-loaded T2 cells (T2/CEA) (n = 4 samples; ****p ≤ 0.0001 compared with untreated day-6 CTLs by one-way ANOVA). E Apoptosis of the CRC antigen-activated CTLs induced by autologous tumor cells preincubated with anti-HLA-I or IgG (n = 4 samples; ****p ≤ 0.0001 compared with IgG by one-way ANOVA). F Apoptosis of the CEA-specific CTLs induced by CEA-loaded T2 cells (T2/CEA) preincubated with anti-HLA-I or IgG (n = 4 samples; ****p ≤ 0.0001 compared with IgG by one-way ANOVA)
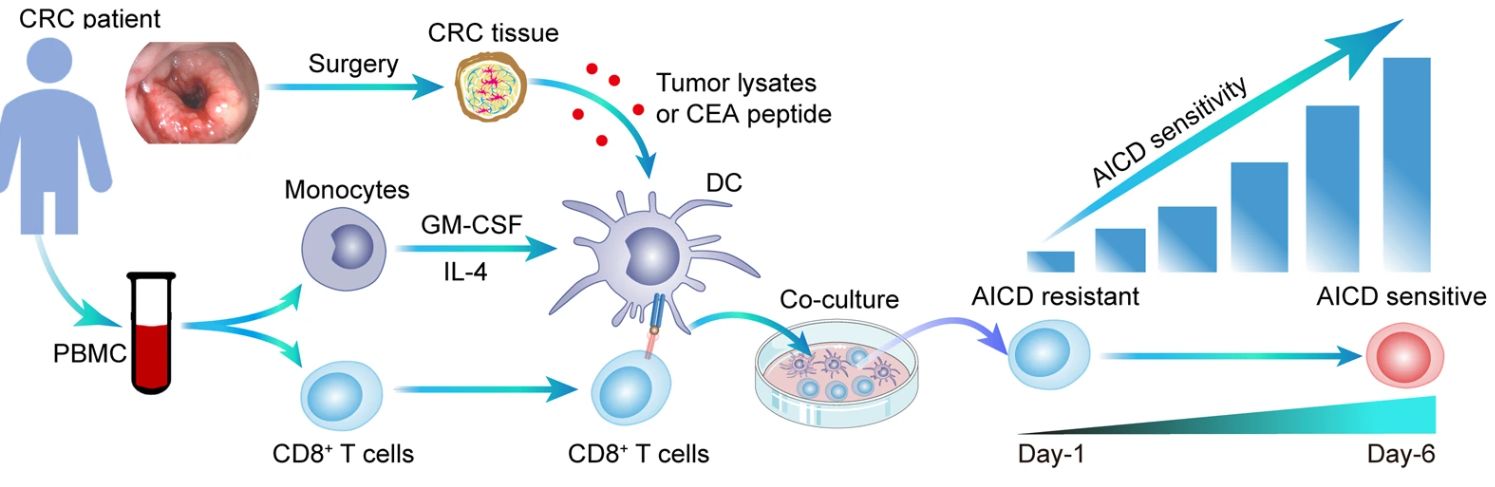
KRAS突变型(KRASMUT)癌症患者通常伴随着肿瘤免疫逃逸,然而潜在机制尚未研究清楚。我们报告了来自KRASMUT癌症的肿瘤特异性细胞毒性T淋巴细胞(CTLs)对激活诱导的细胞死亡(AICD)敏感。circATXN7是一种与核因子-κB(NF-κB)相互作用的环状RNA,通过失活NF-κB来控制T细胞对AICD的敏感性。从机制上讲,源自KRASMUT肿瘤细胞产生的乳酸导致组蛋白乳酸化,直接激活circATXN7的转录,circATXN7结合NF-κBP65蛋白亚单位并掩盖P65蛋白核定位信号基序,从而将其隔离在细胞质中。临床上,在肿瘤特异性CTLs中circATXN7的上调与不良临床结果和免疫治疗耐药相关。在CD8+ T细胞中遗传消除circATXN7导致突变选择性肿瘤抑制,同时还增加了雌性小鼠多种肿瘤模型中抗PD1的有效性。此外,靶向转移的肿瘤反应性CTLs中的circATXN7可以改善其抗肿瘤活性。这些发现为淋巴细胞表达的circRNA如何促进T细胞命运决定和抗癌免疫疗法提供了新的见解。
#联科生物产品助力科研

参考文献:
Zhou, C., Li, W., Liang, Z. et al. Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death. Nat Commun 15, 499 (2024).
原文链接: